

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 10:00
How many grams of cupric sulfate pentahydrate are needed to prepare 50.00 ml of 0.0800m cuso4× 5h2o?
Answers: 3

Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3

You know the right answer?
The combustion of propane (c3h8) produces co2 and h2o: c3h8 (g) 5o2 (g) → 3co2 (g) 4h2o (g) the rea...
Questions






Mathematics, 27.08.2020 23:01


Mathematics, 27.08.2020 23:01





Mathematics, 27.08.2020 23:01







History, 27.08.2020 23:01

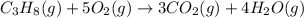
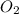 produces = 4 moles of
produces = 4 moles of 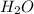
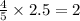 moles of
moles of 

