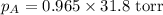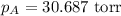Chemistry, 12.07.2019 18:10 damaricoleman42
Calculate the vapor pressure of a solution containing 24.6 g of glycerin (c3h8o3) in 134 ml of water at 30.0 ∘c. the vapor pressure of pure water at this temperature is 31.8 torr. assume that glycerin is not volatile and dissolves molecularly (i. e., it is not ionic) and use a density of 1.00 g/ml for the water.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1

You know the right answer?
Calculate the vapor pressure of a solution containing 24.6 g of glycerin (c3h8o3) in 134 ml of water...
Questions

History, 07.10.2019 13:50

Health, 07.10.2019 13:50

Advanced Placement (AP), 07.10.2019 13:50

History, 07.10.2019 13:50

Geography, 07.10.2019 13:50




Biology, 07.10.2019 13:50

Mathematics, 07.10.2019 13:50


Mathematics, 07.10.2019 13:50

Health, 07.10.2019 13:50

History, 07.10.2019 13:50


Biology, 07.10.2019 13:50

Biology, 07.10.2019 13:50


Physics, 07.10.2019 13:50

English, 07.10.2019 13:50

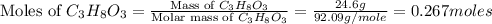
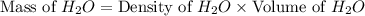
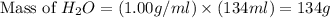
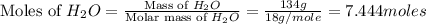
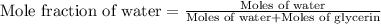
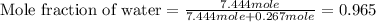
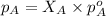
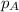 = vapor pressure of solution = ?
= vapor pressure of solution = ?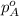 = vapor pressure of pure water= 31.8 torr
= vapor pressure of pure water= 31.8 torr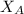 = mole fraction of water = 0.965
= mole fraction of water = 0.965