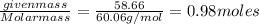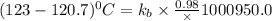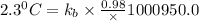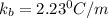Chemistry, 12.07.2019 20:30 joannegrace869
The normal boiling point of a certain liquid x is 120.7°c , but when 58.66g of urea nh22co are dissolved in 950.g of x , it is found that the solution boils at 123.0°c instead. use this information to calculate the molal boiling point elevation constant kb of x .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
The normal boiling point of a certain liquid x is 120.7°c , but when 58.66g of urea nh22co are disso...
Questions

Mathematics, 14.12.2021 08:00

Mathematics, 14.12.2021 08:00

English, 14.12.2021 08:00

Biology, 14.12.2021 08:00

Mathematics, 14.12.2021 08:00

Mathematics, 14.12.2021 08:00

Mathematics, 14.12.2021 08:00



Mathematics, 14.12.2021 08:00


Mathematics, 14.12.2021 08:00





Mathematics, 14.12.2021 08:00

Mathematics, 14.12.2021 08:00


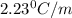
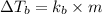
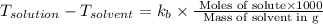
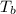 = change in boiling point
= change in boiling point
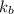 = boiling point constant
= boiling point constant