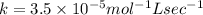Chemistry, 12.07.2019 21:30 mxddisonxo
The reaction between ethyl bromide (c2h5br) and hydroxide ion in ethyl alcohol at 330 k, c2h5br(alc) + oh-(alc) --> c2h5oh(l) + br-(alc), is first order each in ethyl bromide and hydroxide ion. when [c2h5br] is 0.0477 m and [oh-] is 0.100 m, the rate of disappearance of ethyl bromide is 1.7 x 10^-7 m/s.
what is the value of the rate constant?
k=?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
The reaction between ethyl bromide (c2h5br) and hydroxide ion in ethyl alcohol at 330 k, c2h5br(alc)...
Questions

Social Studies, 09.02.2021 22:00


Mathematics, 09.02.2021 22:00

Physics, 09.02.2021 22:00


Biology, 09.02.2021 22:00

Social Studies, 09.02.2021 22:00

History, 09.02.2021 22:00

Advanced Placement (AP), 09.02.2021 22:00

German, 09.02.2021 22:00


Mathematics, 09.02.2021 22:00

Mathematics, 09.02.2021 22:00

Mathematics, 09.02.2021 22:00

Mathematics, 09.02.2021 22:00

Mathematics, 09.02.2021 22:00




Computers and Technology, 09.02.2021 22:00

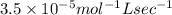
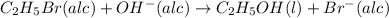
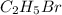 = 1
= 1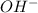 = 1
= 1![Rate=k[C_2H_5Br]^1[OH^-]^1](/tpl/images/0082/3546/7f75e.png)
![Rate=-\frac{1d[C_2H_5Br]}{dt}=k[C_2H_5Br]^1[OH^-]^1](/tpl/images/0082/3546/b56e9.png)
![\frac{d[C_2H_5]}{dt}]=1.7\times 10^{-7}](/tpl/images/0082/3546/5e05d.png)
![Rate=1.7\times 10^{-7}=k[0.0477]^1[0.100]^1](/tpl/images/0082/3546/1123d.png)