
Chemistry, 12.07.2019 23:20 savannahvargas512
Enter your answer in the provided box. one of the half-reactions for the electrolysis of water is 2h2o(l) → o2(g) + 4h+(aq) + 4e− if 3.696 l of o2 is collected at 25°c and 755 mmhg, how many faradays of electricity had to pass through the solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1
You know the right answer?
Enter your answer in the provided box. one of the half-reactions for the electrolysis of water is 2h...
Questions


Mathematics, 02.08.2019 03:30



Mathematics, 02.08.2019 03:30




Mathematics, 02.08.2019 03:30





History, 02.08.2019 03:30


Social Studies, 02.08.2019 03:30


History, 02.08.2019 03:30


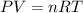
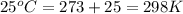
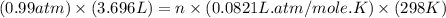
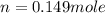
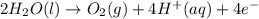
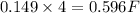 of electricity.
of electricity.

