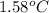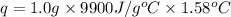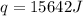Chemistry, 13.07.2019 01:30 jmanrules200
When 1.0 g of fructose, c6h12o6(s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the calorimeter increases by 1.58 °c. if the heat capacity of the calorimeter and its contents is 9.90 kj/°c, what is q for this combustion?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
When 1.0 g of fructose, c6h12o6(s), a sugar commonly found in fruits, is burned in oxygen in a bomb...
Questions


Biology, 11.03.2020 03:31


Mathematics, 11.03.2020 03:31

Biology, 11.03.2020 03:31

Computers and Technology, 11.03.2020 03:31



Geography, 11.03.2020 03:31

Biology, 11.03.2020 03:31




Mathematics, 11.03.2020 03:31

English, 11.03.2020 03:32





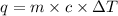
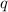 = heat of combustion = ?
= heat of combustion = ?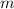 = mass of fructose = 1.0 g
= mass of fructose = 1.0 g = heat capacity of the calorimteter =
= heat capacity of the calorimteter = 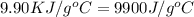
 = change in temperature =
= change in temperature =