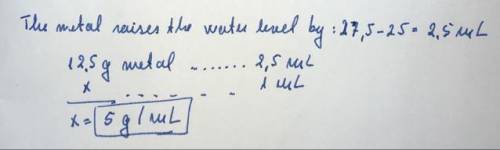Chemistry, 13.07.2019 06:10 dollangellface22
Apiece of metal with a mass of 12.5 g is placed into a graduated cylinder that contains 25.00 ml of water, raising the water level to 27.5 ml. what is the density of the metal? 0.45 g/ml2.23 g/ml4.0 g/ml5.0 g/ml5.50 g/ml

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1
You know the right answer?
Apiece of metal with a mass of 12.5 g is placed into a graduated cylinder that contains 25.00 ml of...
Questions


Chemistry, 09.12.2020 22:00

Physics, 09.12.2020 22:00



Chemistry, 09.12.2020 22:00


English, 09.12.2020 22:00

Mathematics, 09.12.2020 22:00


History, 09.12.2020 22:00

English, 09.12.2020 22:00




Physics, 09.12.2020 22:00


History, 09.12.2020 22:00

Mathematics, 09.12.2020 22:00

Mathematics, 09.12.2020 22:00