
Chemistry, 13.07.2019 22:20 highschoolboy
The fraction of a radioactive isotope remaining at time t is (1/2)^t/t1/2 where t1/2 is the half-life. if the half-life of carbon−14 is 5,730 yr, what fraction of carbon−14 in a piece of charcoal remains after
(a) 14.0 yr?
(b) 1.900 × 10^4 yr? × 10 (enter your answer in scientific notation.)
(c) 1. × 10^5 yr? × 10 (enter your answer in scientific notation.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
The fraction of a radioactive isotope remaining at time t is (1/2)^t/t1/2 where t1/2 is the half-lif...
Questions




Mathematics, 17.10.2020 23:01

English, 17.10.2020 23:01

Mathematics, 17.10.2020 23:01


Social Studies, 17.10.2020 23:01

Mathematics, 17.10.2020 23:01



Mathematics, 17.10.2020 23:01





Mathematics, 17.10.2020 23:01


Mathematics, 17.10.2020 23:01

Mathematics, 17.10.2020 23:01

 fraction of carbon−14 in a piece of charcoal remains after 14.0 years.
fraction of carbon−14 in a piece of charcoal remains after 14.0 years.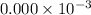 fraction of carbon−14 in a piece of charcoal remains after
fraction of carbon−14 in a piece of charcoal remains after 
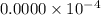 fraction of carbon−14 in a piece of charcoal remains after
fraction of carbon−14 in a piece of charcoal remains after 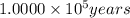 .
.![[A]=\frac{(\frac{1}{2})^t}{t_{\frac{1}{2}}}](/tpl/images/0086/3350/d6525.png)
![\log [A]=t\log[\frac{1}{2}]-\log [t_{\frac{1}{2}}]](/tpl/images/0086/3350/f81b0.png)
 = half life of the carbon−14 =5,730 years
= half life of the carbon−14 =5,730 years![\log [A]= 14 years\times (-3010)-\log [5,730 years]](/tpl/images/0086/3350/51128.png)
![[A]=1.065\times 10^{-8}](/tpl/images/0086/3350/2e677.png)
![\log [A]= 1.900\times 10^4 years\times (-3010)-\log [5,730 years]](/tpl/images/0086/3350/5f99c.png)
![[A]=0.000\times 10^{-3} [/tex](/tpl/images/0086/3350/ae3f2.png)
![\log [A]= 1.0000\times 10^5 years\times (-3010)-\log [5,730 years]](/tpl/images/0086/3350/a2485.png)
![[A]=0.0000\times 10^{-4}](/tpl/images/0086/3350/e1222.png)


