Sue used the following method to convert 5 g/gal to oz/m^3.
((5 g) / (1 gal)) x ((1 gal/ (0.00...

Chemistry, 14.07.2019 21:10 Buxtoman8431
Sue used the following method to convert 5 g/gal to oz/m^3.
((5 g) / (1 gal)) x ((1 gal/ (0.00378541 m^3)) x ((1 m^3) / (28.3495 g)) = 45.59 oz/m^3
(1 gal = 0.00378541 m^3 and 1 oz = 28.3495 g)
what is the error in sue’s conversion method?
a. 0.00378541 m^3 should be written as 0.00378541 gal.
b. 28.3495 g should be written as 28.3495 oz.
c. 5 g should be written as 5 oz.
d. 1 m^3 should be written as 1 oz.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
Questions

Mathematics, 30.01.2020 03:44


Mathematics, 30.01.2020 03:44



Mathematics, 30.01.2020 03:44

English, 30.01.2020 03:44


Mathematics, 30.01.2020 03:44


Mathematics, 30.01.2020 03:44

History, 30.01.2020 03:44





Mathematics, 30.01.2020 03:44

Social Studies, 30.01.2020 03:44

French, 30.01.2020 03:44

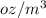
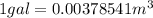
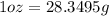
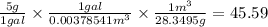 unitless (not possible)
unitless (not possible)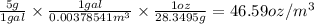
 1 oz should be written.
1 oz should be written.

