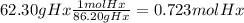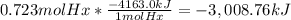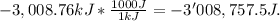Chemistry, 15.07.2019 19:30 wardlawshaliyah
The combustion of hexane is given by the following reaction. 2 c6h14 + 19 o2 12 co2 + 14 h2o the enthalpy of reaction is −4163.0 kj/mol. how much energy (in joules) will be released if 62.30 grams of hexane is burned. (molar mass of hexane = 86.20 g/mol).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
The combustion of hexane is given by the following reaction. 2 c6h14 + 19 o2 12 co2 + 14 h2o the ent...
Questions

History, 30.04.2021 21:50



Mathematics, 30.04.2021 21:50

Mathematics, 30.04.2021 21:50

Mathematics, 30.04.2021 21:50

Social Studies, 30.04.2021 21:50


Mathematics, 30.04.2021 21:50

Computers and Technology, 30.04.2021 21:50







Mathematics, 30.04.2021 21:50



Mathematics, 30.04.2021 21:50