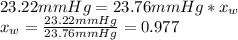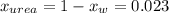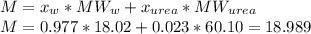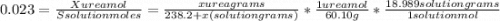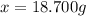Chemistry, 16.07.2019 04:10 reginaldlegette
The vapor pressure of water is 23.76 mm hg at 25°c. how many grams of urea, ch4n2o, a nonvolatile, nonelectrolyte (mw = 60.10 g/mol), must be added to 238.2 grams of water to reduce the vapor pressure to 23.22 mm hg ? water = h2o = 18.02 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

You know the right answer?
The vapor pressure of water is 23.76 mm hg at 25°c. how many grams of urea, ch4n2o, a nonvolatile, n...
Questions



Mathematics, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30


Biology, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30

English, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30


Mathematics, 22.03.2021 18:30



Mathematics, 22.03.2021 18:30

English, 22.03.2021 18:30


Mathematics, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30

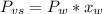
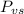 is the vapor pressure of the solution,
is the vapor pressure of the solution, 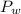 is the vapor pressure of the pure water, and
is the vapor pressure of the pure water, and 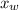 is the molar fraction of water. This equation applies just for that kind of solutes and at low pressures (23.76 mmHg is a low pressure).
is the molar fraction of water. This equation applies just for that kind of solutes and at low pressures (23.76 mmHg is a low pressure).