select one:

Chemistry, 17.07.2019 00:40 savyblue1724707
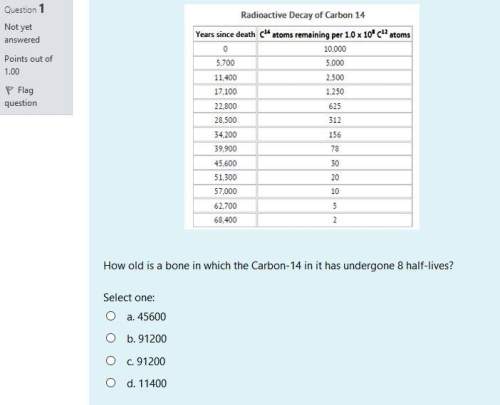
1) how old is a bone in which the carbon-14 in it has undergone 8 half-lives?
select one:
a. 45600
b. 91200
c. 91200
d. 11400
2) in the process of radiocarbon dating, the fixed period of radioactive decay used to determine age is called the
select one:
a. exponent.
b. half-life.
c. isotope.
d. nucleus.
3) a certain byproduct in nuclear reactors, 210po, decays to become 206pb. after a time period of about 276 days, only about 25% of an original sample of 210po remains. the remainder has decayed to 206pb. determine the approximate half-life of 210po.
select one:
a. 138 days
b. 276 days
c. 414 days
d. 552 days

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1


Chemistry, 23.06.2019 09:00
What sources of error may have contributed to the percent yield not being 100 percent? think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.
Answers: 2

Chemistry, 23.06.2019 15:00
An isotope undergoes radioactive decay by emitting radiation that has no mass. what other characteristic does the radiation have?
Answers: 3
You know the right answer?
1) how old is a bone in which the carbon-14 in it has undergone 8 half-lives?
select one:
select one:
Questions



Mathematics, 19.03.2020 22:14


Mathematics, 19.03.2020 22:14











Mathematics, 19.03.2020 22:15






