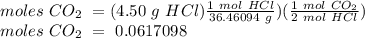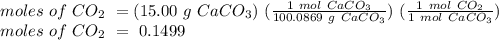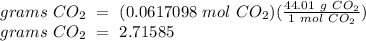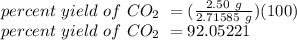If 4.50 g of hcl are reacted with 15.00 g of caco, according to the following balanced chemical equation, calculate the theoretical yield of. co. 2hcl + caco3 cacl2 + h2o + co2 if 2.50 g of co2 are isolated, after carrying out the above reaction, calculate the percent yield of co2.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
If 4.50 g of hcl are reacted with 15.00 g of caco, according to the following balanced chemical equa...
Questions



Spanish, 20.08.2021 01:00

Mathematics, 20.08.2021 01:00

Mathematics, 20.08.2021 01:00

History, 20.08.2021 01:00

English, 20.08.2021 01:00

Mathematics, 20.08.2021 01:00





Mathematics, 20.08.2021 01:00

Mathematics, 20.08.2021 01:00




Biology, 20.08.2021 01:00