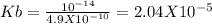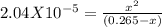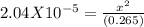Chemistry, 18.07.2019 19:30 sanchez626
Sodium cyanide, nacn, is a salt formed from the neutralization of the weak acid hydrocyanic acid, hcn, with the strong base sodium hydroxide. given that the value of ka for hydrocyanic acid is 4.90×10−10, what is the ph of a 0.265 m solution of sodium cyanide at 25∘c?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

You know the right answer?
Sodium cyanide, nacn, is a salt formed from the neutralization of the weak acid hydrocyanic acid, hc...
Questions



Mathematics, 09.09.2019 20:30





Computers and Technology, 09.09.2019 20:30


Computers and Technology, 09.09.2019 20:30




Computers and Technology, 09.09.2019 20:30






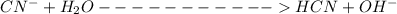
![K=\frac{[HCN][OH^{-}}{[CN^{-}]}](/tpl/images/0105/1012/dad8f.png)