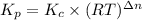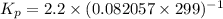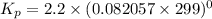Chemistry, 18.07.2019 21:10 GhostElite295
Calculate the equilibrium constant kp for this reaction, given the following information (at 299 k ): 2no(g)+br2(g)⇌2nobr(g)kc=2.2 2no(g)⇌n2(g)+o2(g)kc=2.3×1030

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
Calculate the equilibrium constant kp for this reaction, given the following information (at 299 k )...
Questions


English, 12.02.2021 01:00

Mathematics, 12.02.2021 01:00



Biology, 12.02.2021 01:00

Mathematics, 12.02.2021 01:00

Physics, 12.02.2021 01:00

Social Studies, 12.02.2021 01:00

Geography, 12.02.2021 01:00

Biology, 12.02.2021 01:00

Mathematics, 12.02.2021 01:00




English, 12.02.2021 01:00




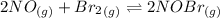 , Kp = 0.08967
, Kp = 0.08967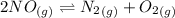 , Kp = 2.3×10³⁰
, Kp = 2.3×10³⁰