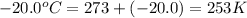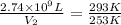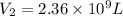Chemistry, 18.07.2019 21:10 krystalhurst97
The natural gas in a storage reservoir, under a pressure of 1.00 atmosphere, has a volume of 2.74 × 109 l at 20.0°c. the temperature at a later date falls to –20.0°c, but the pressure remains constant. calculate the volume that the gas now occupies

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1


Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 23.06.2019 13:30
Which correctly identifies the parts of a transverse wave? a: crest b: amplitude c: wavelength d: trough a: trough b: amplitude c: crest d: wavelength a: trough b: amplitude c: wavelength d: crest a: crest b: amplitude c: trough d: wavelength
Answers: 1
You know the right answer?
The natural gas in a storage reservoir, under a pressure of 1.00 atmosphere, has a volume of 2.74 ×...
Questions


Mathematics, 11.06.2020 22:57

Medicine, 11.06.2020 22:57





Mathematics, 11.06.2020 22:57

Mathematics, 11.06.2020 22:57

Mathematics, 11.06.2020 22:57


Mathematics, 11.06.2020 22:57



Mathematics, 11.06.2020 22:57





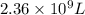
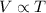
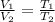
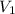 = initial volume of gas =
= initial volume of gas = 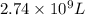
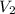 = final volume of gas = ?
= final volume of gas = ?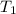 = initial temperature of gas =
= initial temperature of gas = 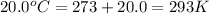
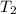 = final temperature of gas =
= final temperature of gas =