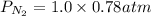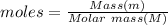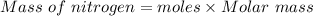Chemistry, 18.07.2019 22:10 legendman27
Calculate the mass of nitrogen dissolved at room temperature in an 86.0 l home aquarium. assume a total pressure of 1.0 atm and a mole fraction for nitrogen of 0.78.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

You know the right answer?
Calculate the mass of nitrogen dissolved at room temperature in an 86.0 l home aquarium. assume a to...
Questions


Geography, 24.09.2019 16:30

History, 24.09.2019 16:30

English, 24.09.2019 16:30

Mathematics, 24.09.2019 16:30

Biology, 24.09.2019 16:30

Computers and Technology, 24.09.2019 16:30


Mathematics, 24.09.2019 16:30


Mathematics, 24.09.2019 16:30

Mathematics, 24.09.2019 16:30


History, 24.09.2019 16:30


Social Studies, 24.09.2019 16:30



Social Studies, 24.09.2019 16:30

Mathematics, 24.09.2019 16:30

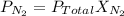
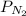 is the partial pressure of nitrogen
is the partial pressure of nitrogen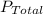 is the Total pressure
is the Total pressure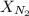 is the mole fraction of nitrogen
is the mole fraction of nitrogen