
Chemistry, 18.07.2019 23:30 kalialee2424
How many grams of nahco3 (fm 84.01 g/mol) should be mixed with na2co3 to produce a 1.00 l buffer solution with ph 9.50. the final concentration of na2co3 in this solution is 0.10 m. pka1 = 6.37 and pka2 = 10.33 for h2co3.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
How many grams of nahco3 (fm 84.01 g/mol) should be mixed with na2co3 to produce a 1.00 l buffer sol...
Questions

Biology, 18.11.2020 15:50


Mathematics, 18.11.2020 15:50

Mathematics, 18.11.2020 15:50

Physics, 18.11.2020 15:50

History, 18.11.2020 15:50

English, 18.11.2020 16:00

Mathematics, 18.11.2020 16:00

History, 18.11.2020 16:00

History, 18.11.2020 16:00

History, 18.11.2020 16:00

Social Studies, 18.11.2020 16:00


Business, 18.11.2020 16:00

Physics, 18.11.2020 16:00

Computers and Technology, 18.11.2020 16:00



Mathematics, 18.11.2020 16:00

History, 18.11.2020 16:00

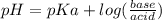
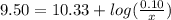
 is the concentration of sodium bicarbonate)
is the concentration of sodium bicarbonate)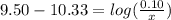
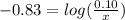
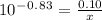
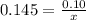
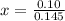
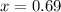
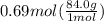
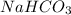 will be required.
will be required. 

