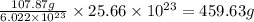Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 23.06.2019 10:30
How is it possible for someone to put an ear to a wall and hear someone in the next room? a.sound waves can travel though solids. b.the waves travel from room to room via air. c.there must be some air in the wall so the sound can travel through it. d.sound waves change to electromagnetic waves and then back again.
Answers: 1
You know the right answer?
Determine how many grams of silver would be produced, if 12.83 x 10^23 atoms of copper react with an...
Questions





English, 13.02.2022 09:30


Mathematics, 13.02.2022 09:30

Mathematics, 13.02.2022 09:30



Social Studies, 13.02.2022 09:30



Mathematics, 13.02.2022 09:40

Computers and Technology, 13.02.2022 09:40

Health, 13.02.2022 09:40

English, 13.02.2022 09:40

Biology, 13.02.2022 09:40

Physics, 13.02.2022 09:40

History, 13.02.2022 09:40

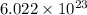 number of atoms.
number of atoms.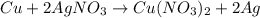
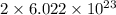 number of atoms of silver.
number of atoms of silver.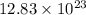 number of atoms of copper will produce =
number of atoms of copper will produce = 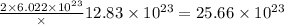 number of atoms of silver.
number of atoms of silver.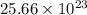 number of atoms will occupy =
number of atoms will occupy =