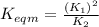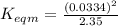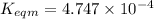Chemistry, 19.07.2019 04:30 yarielisr18
Use the reactions below and their equilibrium constants to predict the equilibrium constant for the reaction 2a(s)⇌3d(g). a(s) ⇌ 12 b(g)+c(g), k1=0.0334 3d(g) ⇌ b(g)+2c(g), k2=2.35

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2
You know the right answer?
Use the reactions below and their equilibrium constants to predict the equilibrium constant for the...
Questions



Mathematics, 07.01.2020 05:31


Physics, 07.01.2020 05:31


Mathematics, 07.01.2020 05:31

Mathematics, 07.01.2020 05:31






Mathematics, 07.01.2020 05:31


Mathematics, 07.01.2020 05:31

Mathematics, 07.01.2020 05:31



Physics, 07.01.2020 05:31


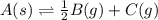
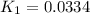
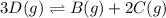

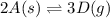
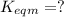
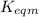 for the final reaction.
for the final reaction.