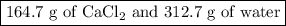Chemistry, 19.07.2019 05:20 kailahgranger
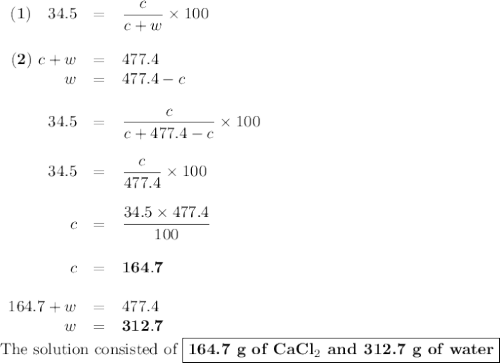
Asolution of cacl2 in water forms a mixture that is 34.5% calcium chloride by mass. if the total mass of the mixture is 477.4 g, what masses of cacl2 and water were used?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
Asolution of cacl2 in water forms a mixture that is 34.5% calcium chloride by mass. if the total mas...
Questions


English, 16.10.2021 22:50



History, 16.10.2021 22:50

Mathematics, 16.10.2021 22:50



Social Studies, 16.10.2021 22:50


World Languages, 16.10.2021 22:50



English, 16.10.2021 22:50

Advanced Placement (AP), 16.10.2021 22:50



Mathematics, 16.10.2021 22:50

History, 16.10.2021 22:50

Geography, 16.10.2021 23:00