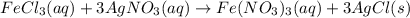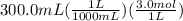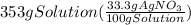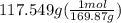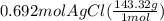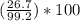Chemistry, 19.07.2019 20:10 laurielaparr2930
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass % solution of silver (u) nitrate and water. 267 grams of a solid precipitate forms. what is the percent yield of the reaction assuming that the solubility of the solid precipitate in water is negligible.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

You know the right answer?
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass %...
Questions


Computers and Technology, 30.09.2019 21:00

Mathematics, 30.09.2019 21:00

Chemistry, 30.09.2019 21:00



Mathematics, 30.09.2019 21:00

Biology, 30.09.2019 21:00

History, 30.09.2019 21:00

Mathematics, 30.09.2019 21:00

History, 30.09.2019 21:00






Chemistry, 30.09.2019 21:00


Biology, 30.09.2019 21:00