Chemistry, 19.07.2019 21:30 btcastongia
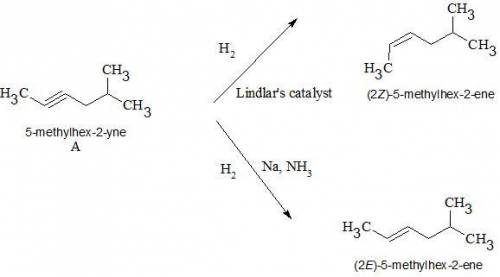
An achiral hydrocarbon a of molecular formula c7h12 reacts with two equivalents of h2 in the presence of pd-c to form ch3ch2ch2ch2ch(ch3)2. one oxidative cleavage product formed by the treatment of a with o3 is ch3cooh. reaction of a with h2 and lindlar catalyst forms b, and reaction of a with na, nh3 forms c. identify compounds a, b, and c. be sure to answer all parts.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
An achiral hydrocarbon a of molecular formula c7h12 reacts with two equivalents of h2 in the presenc...
Questions

History, 09.09.2019 21:20



Mathematics, 09.09.2019 21:20


Mathematics, 09.09.2019 21:20


Chemistry, 09.09.2019 21:20

Mathematics, 09.09.2019 21:20







Chemistry, 09.09.2019 21:20




Mathematics, 09.09.2019 21:20