Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
Calculate the change in entropy that occurs in the system when 4.20 mole of diethyl ether (\(\rm c_4...
Questions


Spanish, 08.07.2019 22:50

Chemistry, 08.07.2019 22:50

Social Studies, 08.07.2019 22:50





Advanced Placement (AP), 08.07.2019 22:50


Chemistry, 08.07.2019 22:50


Biology, 08.07.2019 22:50

Social Studies, 08.07.2019 22:50

Biology, 08.07.2019 22:50


Physics, 08.07.2019 22:50


Advanced Placement (AP), 08.07.2019 22:50

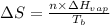
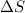 = entropy change of the system = ?
= entropy change of the system = ?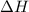 = enthalpy of vaporization = 34.6 kJ/mole
= enthalpy of vaporization = 34.6 kJ/mole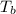 = normal boiling point =
= normal boiling point =