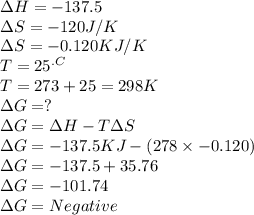Chemistry, 19.07.2019 22:20 haleybain6353
C2h4(g) + h2(g) → c2h6(g) δh = –137.5 kj; δs = –120.5 j/k calculate δg at 25 °c and determine whether the reaction is spontaneous. does δg become more negative or more positive as the temperature increases?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 02:30
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
C2h4(g) + h2(g) → c2h6(g) δh = –137.5 kj; δs = –120.5 j/k calculate δg at 25 °c and determine wheth...
Questions

Mathematics, 04.07.2019 18:40

Physics, 04.07.2019 18:40

Mathematics, 04.07.2019 18:40





Mathematics, 04.07.2019 18:40

Mathematics, 04.07.2019 18:40


Mathematics, 04.07.2019 18:40

English, 04.07.2019 18:40

Mathematics, 04.07.2019 18:40



Mathematics, 04.07.2019 18:40

Mathematics, 04.07.2019 18:40