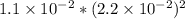Chemistry, 19.07.2019 23:20 jasmine3051
Excess ca(oh)2 is shaken with water to produce a saturated solution. the solution is filtered, and a 50.00 ml sample titrated with hcl requires 11.22 ml of 0.0983 m hcl to reach the end point. part a calculate ksp for ca(oh)2.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:50
What does standard deviation reveal about data? a. the average of all the data points b. which of the data points is most reliable c. how spread out the data points are d. the percent error included in the data
Answers: 2

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
Excess ca(oh)2 is shaken with water to produce a saturated solution. the solution is filtered, and a...
Questions



Mathematics, 16.12.2020 23:10

Physics, 16.12.2020 23:10

Mathematics, 16.12.2020 23:10



Mathematics, 16.12.2020 23:10








Mathematics, 16.12.2020 23:10


English, 16.12.2020 23:10

Mathematics, 16.12.2020 23:10

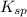 for calcium hydroxide is
for calcium hydroxide is 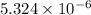
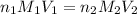
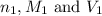 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 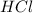
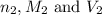 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 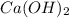
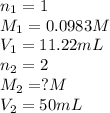
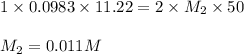
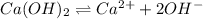
![K_{sp}=[Ca^{2+}][OH^-]^2](/tpl/images/0109/5708/8de55.png)
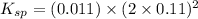
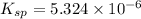
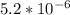
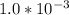
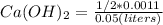
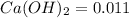 .
.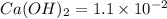 .
.