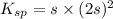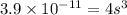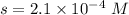What is the molar solubility of calcium fluoride ( caf2) in water? the solubility-product constant for caf2 is 3.9 ⋅ 10-11 at 25 °c. what is the molar solubility of calcium fluoride ( caf2) in water? the solubility-product constant for caf2 is 3.9 10-11 at 25 °c. 3.4 ⋅ 10-4 8.8 ⋅ 10-6 3.1 ⋅ 10-6 1.3 ⋅ 10-11 2.1 ⋅ 10-4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

You know the right answer?
What is the molar solubility of calcium fluoride ( caf2) in water? the solubility-product constant...
Questions

Mathematics, 17.05.2020 02:57



Biology, 17.05.2020 02:57

Mathematics, 17.05.2020 02:57




Geography, 17.05.2020 02:57


Mathematics, 17.05.2020 02:57




Advanced Placement (AP), 17.05.2020 02:57


Biology, 17.05.2020 02:57

Mathematics, 17.05.2020 02:57

History, 17.05.2020 02:57

Mathematics, 17.05.2020 02:57

![K_{sp}=\left[Ca^{2+} \right]\left[F^{-} \right]^2](/tpl/images/0110/5296/1ebe5.png)