
Consider the following equation.
fe2o3(s) + 3h2(g) → 2fe(s) + 3h2o(g)
h = 98.8 kj, and s = 141.5 j/k. is this reaction spontaneous or nonspontaneous at high and low temperatures?
a. spontaneous at high temperatures, non-spontaneous at low temperatures
b. non-spontaneous at high and low temperatures
c. spontaneous at low temperatures, non-spontaneous at high temperatures
d. spontaneous at high and low temperatures

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1


Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
Consider the following equation.
fe2o3(s) + 3h2(g) → 2fe(s) + 3h2o(g)
h = 98.8 kj, and s...
fe2o3(s) + 3h2(g) → 2fe(s) + 3h2o(g)
h = 98.8 kj, and s...
Questions


Chemistry, 03.06.2020 19:58

Mathematics, 03.06.2020 19:58

English, 03.06.2020 19:58


Mathematics, 03.06.2020 19:58

Geography, 03.06.2020 19:58

Mathematics, 03.06.2020 19:58


Mathematics, 03.06.2020 19:58


Mathematics, 03.06.2020 19:59

History, 03.06.2020 19:59

Mathematics, 03.06.2020 19:59


Biology, 03.06.2020 19:59


Physics, 03.06.2020 19:59


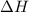 and the entropy change
and the entropy change 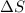 due to this reaction are positive. A chemical reaction will be spontaneous only if the change in its Gibbs Free Energy
due to this reaction are positive. A chemical reaction will be spontaneous only if the change in its Gibbs Free Energy 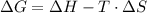 is negative.
is negative. 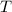 is the absolute temperature in degrees Kelvins.
is the absolute temperature in degrees Kelvins.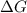 will initially be close to
will initially be close to 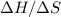 . The reaction will eventually become spontaneous.
. The reaction will eventually become spontaneous.

