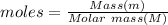Chemistry, 22.07.2019 16:10 MannyBanko1350
Methanol, ethanol, and n−propanol are three common alcohols. when 1.00 g of each of these alcohols is burned in air, heat is liberated as indicated. calculate the heats of combustion of these alcohols in kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2
You know the right answer?
Methanol, ethanol, and n−propanol are three common alcohols. when 1.00 g of each of these alcohols i...
Questions


Computers and Technology, 12.02.2020 00:56




Mathematics, 12.02.2020 00:56




History, 12.02.2020 00:56



Mathematics, 12.02.2020 00:56



Mathematics, 12.02.2020 00:56



English, 12.02.2020 00:56