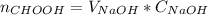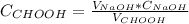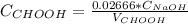Chemistry, 22.07.2019 20:30 fernandoramirez086
3. a student titrated 25.00 ml of a solution of formic acid with sodium hydroxide of known concentration. the student plotted ph verses volume (ml) of naoh added and found that the titration required 26.66 ml of naoh to reach equivalence point. describe how you would use the students’ titration curve to determine the concentration of the formic acid solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
3. a student titrated 25.00 ml of a solution of formic acid with sodium hydroxide of known concentra...
Questions

History, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00

Health, 27.07.2021 14:00

Chemistry, 27.07.2021 14:00





History, 27.07.2021 14:00

English, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00

Chemistry, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00


English, 27.07.2021 14:00