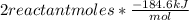Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Consider the reaction h2(g) + cl2(g) → 2hcl(g)δh = −184.6 kj / mol if 2.00 moles of h2 react with 2....
Questions


English, 19.03.2021 18:10


Mathematics, 19.03.2021 18:10

History, 19.03.2021 18:10


Mathematics, 19.03.2021 18:10


Health, 19.03.2021 18:10


Mathematics, 19.03.2021 18:10

Mathematics, 19.03.2021 18:10

History, 19.03.2021 18:10






Mathematics, 19.03.2021 18:10

Mathematics, 19.03.2021 18:10