
Chemistry, 22.07.2019 21:30 Christsflower601
What is the molarity of sodium ions in a solution prepared by diluting 250. ml of 0.550 m na2so4 to 1.25 l? (a) 0.110 m (c) 0.220 m (b) 0.138 m (d) 0.275 m

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2
You know the right answer?
What is the molarity of sodium ions in a solution prepared by diluting 250. ml of 0.550 m na2so4 to...
Questions






History, 05.03.2020 07:46

Computers and Technology, 05.03.2020 07:46



Mathematics, 05.03.2020 07:46






Physics, 05.03.2020 07:48

Mathematics, 05.03.2020 07:48


Mathematics, 05.03.2020 07:49

 =
= 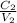
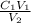
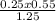 = 0.11M
= 0.11M

