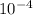Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1


Chemistry, 23.06.2019 07:30
Can you guys answer these questions i need it before 1: 00pm
Answers: 3

Chemistry, 23.06.2019 08:30
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1
You know the right answer?
Calculate the molar solubility of ag2 cro4 in water. use 1.10 x 10-12 as the solubility product cons...
Questions

English, 19.05.2020 03:11


History, 19.05.2020 03:11

Biology, 19.05.2020 03:11


Chemistry, 19.05.2020 03:11


History, 19.05.2020 03:11


English, 19.05.2020 03:11

English, 19.05.2020 03:11

Advanced Placement (AP), 19.05.2020 03:11

Mathematics, 19.05.2020 03:11






History, 19.05.2020 03:11

![s=\sqrt[3]{(1.10)(10^{-12})}=1.03](/tpl/images/0122/2625/96243.png) ×
×