
Atmospheric pressure at sea level is 760 mm hg, and oxygen makes up 20.9% of this air when it is dry. scientists at the mt. washington observatory in new hampshire measured the atmospheric pressure at the summit of mt. washington (6,289 feet above sea level) as 609 mm hg. when the air is dry, the partial pressure of oxygen at the summit is approximately mm hg.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1

Chemistry, 23.06.2019 10:30
Amethod of separation that employs a system with two phases of matter, a mobile phase and a stationary phase, is called
Answers: 2

Chemistry, 23.06.2019 11:30
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3

Chemistry, 23.06.2019 15:10
Certain types of organisms such as fireflies and anglerfish can produce light through chemical reactions in a process called bioluminescence. what kind of chemical reactions occur during bioluminescence? o a) exothermic ob) endothermic oc) recomposition od) decomposition
Answers: 2
You know the right answer?
Atmospheric pressure at sea level is 760 mm hg, and oxygen makes up 20.9% of this air when it is dry...
Questions


Physics, 16.12.2020 06:10

Mathematics, 16.12.2020 06:10

Physics, 16.12.2020 06:10

Mathematics, 16.12.2020 06:10

Mathematics, 16.12.2020 06:10

History, 16.12.2020 06:10



History, 16.12.2020 06:10



Mathematics, 16.12.2020 06:10

History, 16.12.2020 06:10

Business, 16.12.2020 06:10




Mathematics, 16.12.2020 06:10

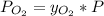
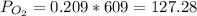 mmHg.
mmHg.

