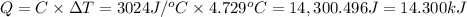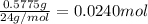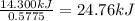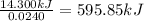A0.5775−g sample of solid magnesium is burned in a constant-volume bomb calorimeter that has a heat capacity of 3024 j/°c. the temperature increases by 4.729°c. (a) calculate the heat associated with the burning mg in kj/g. kj/g (b) calculate the heat associated with the burning of mg in kj/mol. kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2
You know the right answer?
A0.5775−g sample of solid magnesium is burned in a constant-volume bomb calorimeter that has a heat...
Questions

Mathematics, 25.02.2021 18:40

Mathematics, 25.02.2021 18:40





English, 25.02.2021 18:40

Mathematics, 25.02.2021 18:40

Geography, 25.02.2021 18:40

Mathematics, 25.02.2021 18:40

Chemistry, 25.02.2021 18:40



Mathematics, 25.02.2021 18:40


Mathematics, 25.02.2021 18:40


History, 25.02.2021 18:40


Engineering, 25.02.2021 18:40