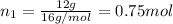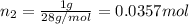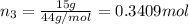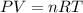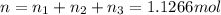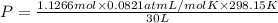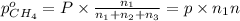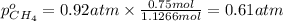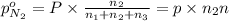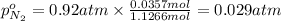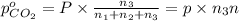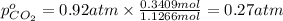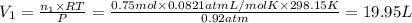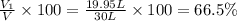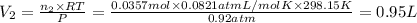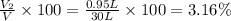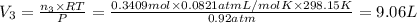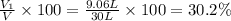Chemistry, 24.07.2019 16:10 jacksonhoyt8049
A30-liter volume of gas at 25°c contains 12 g of methane, 1 g of nitrogen, and 15 g of carbon dioxide. calculate (a) the moles of each gas present, (b) the partial pressure exerted by each gas, (c) the total pressure exerted by the mixture, and (d) the percentage by volume of each gas in the mixture. you may assume ideal gas behavior

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
A30-liter volume of gas at 25°c contains 12 g of methane, 1 g of nitrogen, and 15 g of carbon dioxid...
Questions



French, 21.08.2019 08:20


Health, 21.08.2019 08:20

History, 21.08.2019 08:20




Mathematics, 21.08.2019 08:20


Social Studies, 21.08.2019 08:20

English, 21.08.2019 08:20


Mathematics, 21.08.2019 08:20

Health, 21.08.2019 08:20

Biology, 21.08.2019 08:20

Mathematics, 21.08.2019 08:20

Health, 21.08.2019 08:20

Physics, 21.08.2019 08:20