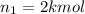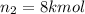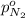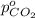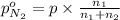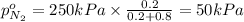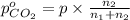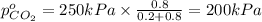Chemistry, 24.07.2019 19:10 holasoykawaii10
Arigid tank is divided into two equal volumes. one side contains 2 kmol of nitrogen n2 at 500 kpa while the other side contains 8 kmol of co2 at 200 kpa. the two sides are now connected and the gases are mixed and forming a homogeneous mixture at 250 kpa. find the partial pressure of the co2 in the final mixture.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
Arigid tank is divided into two equal volumes. one side contains 2 kmol of nitrogen n2 at 500 kpa wh...
Questions




Mathematics, 12.06.2021 01:30


Business, 12.06.2021 01:30


Mathematics, 12.06.2021 01:30


Mathematics, 12.06.2021 01:30


Mathematics, 12.06.2021 01:30

Mathematics, 12.06.2021 01:30


Mathematics, 12.06.2021 01:30

Health, 12.06.2021 01:30


Mathematics, 12.06.2021 01:30


Mathematics, 12.06.2021 01:30

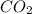 in the final mixture is 200 kPa.
in the final mixture is 200 kPa.