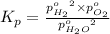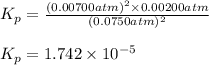Chemistry, 25.07.2019 01:20 jaymee2904p88tgh
The elementary reaction 2h2o(g)↽−−⇀2h2(g)+o2(g) proceeds at a certain temperature until the partial pressures of h2o, h2, and o2 reach 0.0750 atm, 0.00700 atm, and 0.00200 atm, respectively. what is the value of the equilibrium constant at this temperature?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

You know the right answer?
The elementary reaction 2h2o(g)↽−−⇀2h2(g)+o2(g) proceeds at a certain temperature until the partial...
Questions

Mathematics, 07.01.2020 22:31




Mathematics, 07.01.2020 22:31

History, 07.01.2020 22:31


History, 07.01.2020 22:31




Mathematics, 07.01.2020 22:31

Social Studies, 07.01.2020 22:31


Mathematics, 07.01.2020 22:31


Chemistry, 07.01.2020 22:31



 is the value of the equilibrium constant at this temperature.
is the value of the equilibrium constant at this temperature.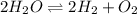
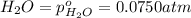
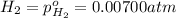
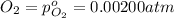
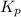 for the given chemical equation is:
for the given chemical equation is: