

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3


Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) a 0.105 l sample of...
Questions


History, 06.01.2020 08:31

History, 06.01.2020 08:31

Mathematics, 06.01.2020 08:31

Biology, 06.01.2020 08:31

Spanish, 06.01.2020 08:31





Biology, 06.01.2020 08:31



Mathematics, 06.01.2020 08:31

Mathematics, 06.01.2020 08:31



Mathematics, 06.01.2020 08:31

Mathematics, 06.01.2020 08:31

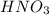 comes out to be 0.088 M.
comes out to be 0.088 M.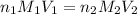
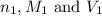 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 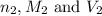 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 




