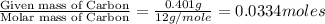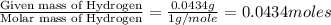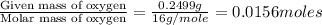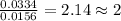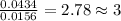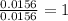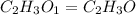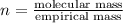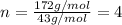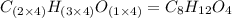Chemistry, 25.07.2019 03:10 paytonpaige22
When 0.6943 g of a compound is subjected to combustion analysis it produced 1.471 g co2 and 0.391 g h2o. what is its empirical and molecular formula if its molar mass is 172 g/mol if the compound is composed of only carbon, hydrogen, and oxygen.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Why do you suppose the structural polysaccharide cellulose does not contain branches? why do you suppose the structural polysaccharide cellulose does not contain branches? branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby decreasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby increasing the rigidity and strength of the microfibrils. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby increasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby decreasing the rigidity and strength of the microfibrils.
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
When 0.6943 g of a compound is subjected to combustion analysis it produced 1.471 g co2 and 0.391 g...
Questions

Physics, 02.09.2019 19:30


Mathematics, 02.09.2019 19:30

Advanced Placement (AP), 02.09.2019 19:30




Geography, 02.09.2019 19:30


Health, 02.09.2019 19:30




World Languages, 02.09.2019 19:30


Chemistry, 02.09.2019 19:30

Social Studies, 02.09.2019 19:30

Mathematics, 02.09.2019 19:30

Mathematics, 02.09.2019 19:30

Chemistry, 02.09.2019 19:30

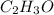 and
and 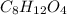 respectively.
respectively.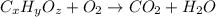
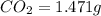
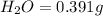
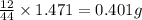 of carbon will be contained.
of carbon will be contained.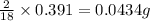 of hydrogen will be contained.
of hydrogen will be contained.