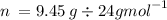Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
Mg metal reacts with hcl to produce hydrogen gas. mg(s)+2hcl(aq)→mgcl2(aq)+h2(g) part a what volume...
Questions




English, 22.01.2021 16:00

English, 22.01.2021 16:10



Mathematics, 22.01.2021 16:10




Social Studies, 22.01.2021 16:10

Biology, 22.01.2021 16:10

Mathematics, 22.01.2021 16:10



Social Studies, 22.01.2021 16:10



Mathematics, 22.01.2021 16:10