
Chemistry, 25.07.2019 20:20 NatalieAllen11
Enter your answer in the provided box. pentaborane−9 (b5h9) is a colorless, highly reactive liquid that will burst into flames when exposed to oxygen. the reaction is 2b5h9(l) 12o2(g) → 5b2o3(s) 9h2o(l) calculate the kilojoules of heat released per gram of the compound reacted with oxygen. the standard enthalpy of formations of b5h9(l), b2o3(s), and h2o(l) are 73.2, −1271.94, and −285.83 kj/mol, respectively.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Monkeys and bats have similar bone structure in their forelimbs. however, monkeys have longer forelimbs to use for climbing and swinging in trees. bats have shorter forelimbs to use for flight. which term best describes how monkey and bat forelimbs are related to each other? a. homologous b. embryonic c. analogous d. vestigial
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
Enter your answer in the provided box. pentaborane−9 (b5h9) is a colorless, highly reactive liquid t...
Questions

Mathematics, 02.09.2019 08:10


History, 02.09.2019 08:10



Biology, 02.09.2019 08:10


Mathematics, 02.09.2019 08:10

Mathematics, 02.09.2019 08:10

History, 02.09.2019 08:10

Mathematics, 02.09.2019 08:10

Biology, 02.09.2019 08:10

English, 02.09.2019 08:10



Chemistry, 02.09.2019 08:10

World Languages, 02.09.2019 08:10



English, 02.09.2019 08:10

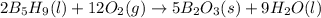
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0132/3420/76c37.png)
![\Delta H=[(n_{H_2O}\times \Delta H_{H_2O})+(n_{B_2O_3}\times \Delta H_{B_2O_3})]-[(n_{B_5H_9}\times \Delta H_{B_5H_9})+(n_{O_2}\times \Delta H_{O_2})]](/tpl/images/0132/3420/48532.png)
![\Delta H=[(9\times -285.83)+(5\times -1271.94)]-[(2\times 73.2)+(12\times 0)]\\\\\Delta H=-9078.57kJ](/tpl/images/0132/3420/9a745.png)
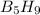 has 63.12 grams of mass
has 63.12 grams of mass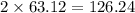 grams of mass
grams of mass


