Chemistry, 25.07.2019 22:20 nataluarenhg6924
The amount of i−3(aq) in a solution can be determined by titration with a solution containing a known concentration of s2o2−3(aq) (thiosulfate ion). the determination is based on the net ionic equation 2s2o2−3(aq)+i3(aq)⟶s4o2−6(aq)+3i−(a q) given that it requires 29.6 ml of 0.260 m na2s2o3(aq) to titrate a 30.0 ml sample of i−3(aq), calculate the molarity of i−3(aq) in the solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
The amount of i−3(aq) in a solution can be determined by titration with a solution containing a know...
Questions



English, 30.03.2020 21:46

Mathematics, 30.03.2020 21:46

Mathematics, 30.03.2020 21:46


English, 30.03.2020 21:46



Engineering, 30.03.2020 21:46

Mathematics, 30.03.2020 21:46

Chemistry, 30.03.2020 21:47


Mathematics, 30.03.2020 21:47

Mathematics, 30.03.2020 21:47

Mathematics, 30.03.2020 21:47




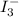 in the solution is, 0.128 M
in the solution is, 0.128 M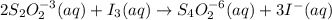
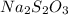 .
.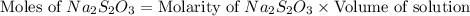
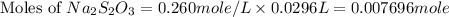
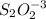 react with 1 mole of
react with 1 mole of 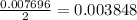 mole of
mole of