Enter the net ionic equation for the reaction of agno3(aq)
with k2so4(aq)
. include phas...

Chemistry, 25.07.2019 23:10 teionamwhite2262
Enter the net ionic equation for the reaction of agno3(aq)
with k2so4(aq)
. include phases. refer to the solubility rules as necessary.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
Questions

Mathematics, 04.07.2019 21:30

History, 04.07.2019 21:30

Geography, 04.07.2019 21:30


Biology, 04.07.2019 21:30

Arts, 04.07.2019 21:30

Mathematics, 04.07.2019 21:30


History, 04.07.2019 21:30

English, 04.07.2019 21:30

Social Studies, 04.07.2019 21:30

Mathematics, 04.07.2019 21:30


Mathematics, 04.07.2019 21:30


Mathematics, 04.07.2019 21:30

Chemistry, 04.07.2019 21:30


English, 04.07.2019 21:30

Computers and Technology, 04.07.2019 21:30

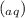 + K₂SO₄
+ K₂SO₄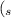 )
)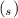 + 2K⁺ + NO₃⁻
+ 2K⁺ + NO₃⁻

