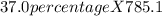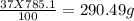Chemistry, 26.07.2019 03:10 mithydizon1553
Asolution of cacl2 in water forms a mixture that is 37.0% calcium chloride by mass. if the total mass of the mixture is 785.1 g, what masses of cacl2 and water were used?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1


Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Asolution of cacl2 in water forms a mixture that is 37.0% calcium chloride by mass. if the total mas...
Questions

Computers and Technology, 28.02.2021 08:00




Business, 28.02.2021 08:00

Biology, 28.02.2021 08:00

Mathematics, 28.02.2021 08:00

Health, 28.02.2021 08:00

Arts, 28.02.2021 08:00


English, 28.02.2021 08:00


Social Studies, 28.02.2021 08:00


Geography, 28.02.2021 08:00


Spanish, 28.02.2021 08:00


Social Studies, 28.02.2021 08:00

Arts, 28.02.2021 08:00