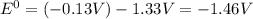Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1


Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
Use tabulated standard electrode potentials to calculate the standard cell potential for the followi...
Questions





Biology, 18.07.2019 06:30


Physics, 18.07.2019 06:30

History, 18.07.2019 06:30



Mathematics, 18.07.2019 06:30

Mathematics, 18.07.2019 06:30

History, 18.07.2019 06:30

History, 18.07.2019 06:30




Mathematics, 18.07.2019 06:30

Mathematics, 18.07.2019 06:30

History, 18.07.2019 06:30

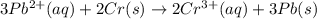
![E^0_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/0133/7776/82211.png)
![E^0_{[Cr^{3+}/Cr]}=1.33V](/tpl/images/0133/7776/e4046.png)
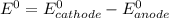
![E^0=E^0_{[Pb^{2+}/Pb]}-E^0_{[Cr^{3+}/Cr]}](/tpl/images/0133/7776/e8ef0.png)