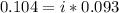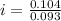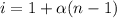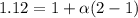Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1


Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Asolution is made by dissolving 0.0500 mol of hf in 1.00 kg of water. the solution was found to free...
Questions




English, 23.06.2019 21:00






Health, 23.06.2019 21:00



History, 23.06.2019 21:00


Geography, 23.06.2019 21:00

English, 23.06.2019 21:00

Mathematics, 23.06.2019 21:00



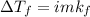
 =0.104 degree C
=0.104 degree C