
Chemistry, 26.07.2019 20:30 joeblaszak4776
Three kilograms of steam is contained in a horizontal, frictionless piston and the cylinder is heated at a constant pressure of 0.5 bar from 100 °c to such a temperature that the specific volume increases by 2.5 times. if the amount of heat that must be added to accomplish this change is 500 kj, calculate the final temperature of the steam, the expansion work, and the change in internal energy.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
Three kilograms of steam is contained in a horizontal, frictionless piston and the cylinder is heate...
Questions




History, 14.04.2020 21:24










Computers and Technology, 14.04.2020 21:24






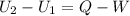
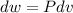
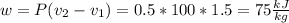 (There is a multiplication by 100 due to the conversion of bar to kPa)
(There is a multiplication by 100 due to the conversion of bar to kPa)
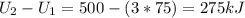
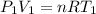
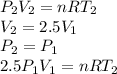
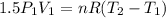
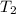 :
: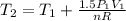
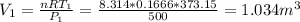
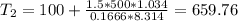 ºC
ºC

