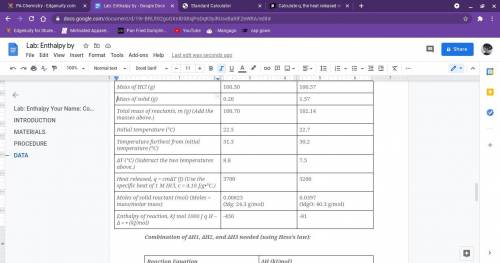
Calculate q, the heat released in each reaction.
use the equation q = cmåt.
(use c = 4.1...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
Questions


Mathematics, 15.07.2020 01:01


Mathematics, 15.07.2020 01:01


Mathematics, 15.07.2020 01:01

Computers and Technology, 15.07.2020 01:01



Mathematics, 15.07.2020 01:01

Mathematics, 15.07.2020 01:01


Mathematics, 15.07.2020 01:01

Mathematics, 15.07.2020 01:01

Mathematics, 15.07.2020 01:01

Mathematics, 15.07.2020 01:01




Mathematics, 15.07.2020 01:01