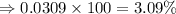Chemistry, 27.07.2019 01:20 ValeryGi3721
"a sample of silicon has an average atomic mass of 28.084amu. in the sample, there are three isotopic forms of silicon. about 92.22% of silicon atoms are 27.9769amu, which have the mass of 28si; about 4.68% are 28.9764amu, which have the mass of 29si, and the remaining isotope, 30si, has a mass of 29.9737amu. calculate the percent isotopic composition of 30si."

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
"a sample of silicon has an average atomic mass of 28.084amu. in the sample, there are three isotopi...
Questions




















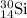 isotope is 3.09 %.
isotope is 3.09 %.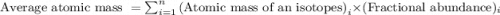 .....(1)
.....(1)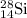 isotope be 'x'
isotope be 'x'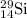 isotope:
isotope:![28.084=[(27.9769\times 0.9222)+(28.9764\times 0.0468)+(29.9737\times x)]\\\\x=0.0309](/tpl/images/0136/9885/23b7c.png)